| Tribolium stock maintenance |

|
| Tribolium:Home |
|
BEETLE WRANGLING TIPS R. W. Beeman, S. Haas & K. Friesen
Index
A. Rearing and Subculturing1.Media- Beetles may be reared in almost any wheat flour, but for optimal results, organic (or some form of pesticide free) whole wheat flour is recommended. The flour may be nutritionally supplemented by adding 5% (by weight) brewer's yeast. To make it easier to separate beetles from the flour mixture, the new flour can be pre-sifted to eliminate the larger sized particles of bran and yeast. Pre-sift with a sieve of the same mesh size ( or smaller) that you use for sifting out adults. (For example, a #25 brass sieve is often used to sift out adults, pupae and large larvae from the flour mixture. Pre-sift the flour with this size of sieve, or an even smaller #30 mesh sieve.) For collecting eggs, a #50 mesh sieve should be used to pre-sift the flour. 2.Containers- Glass containers are the best to use for rearing beetles. The adults and larvae can't climb up the wall of a clean glass jar so you won't loose beetles by having them climb out and walk or crawl away! The container needs to have a lid which will allow air into the jar, but prevent beetles from flying out (or in). Canning jars can be used, with screen inserts and/or filter papers substituted for the solid metal inserts used with the band lids. Other jars such as food jars, or even drinking glasses may be used. Metal lids must have air holes punched in them, and a paper or cloth insert is recommended. Drinking glasses may be covered with paper towels or cloth held on with rubber bands. 3.Starting a stock- Starting a stock from a few (or many) beetles is as simple as putting them in a jar of flour and keeping them at a temperature near 30o C / 86o F (see "Developmental Rates" section below). For about the first six weeks, the beetles should be transferred to new flour by sifting the adults out (see sifting section below) and placing them in a jar of new flour about every other week until the first jars they were in have a thriving adult beetle population. Once there is at least one jar containing lots of adult beetles on the surface, you can (and should) begin subculturing by the paper transfer method (see below). Eventually, you will have older jars of beetles that you want to dispose of and should put them in a freezer for 24 hours before discarding the contents. Disposing of the frozen beetles and dirty flour in a plastic or paper bag is recommended to minimize exposure to the dust. 4.Paper transfer- The use of paper strips to transfer adult beetles from an older stock jar to a new one is the quickest and easiest transfer method. Paper strips approximately 5" X 1" are used for subculturing pint and quart jars. In a bottle with many beetles on the surface of the flour, put the paper strip into the mass of beetles, and wait for them to cover the bottom 1/4 - 1/3 of the strip. Then quickly but carefully withdraw the strip from the first jar and insert it into the jar of new flour. Shake the paper strip and tap its edge against the sides of the jar to remove the beetles. (If the jar has relatively few adults on top, tilt the jar slightly to one side to concentrate the beetles. Adults will gather on the low side, where you can collect them on the paper strip.) Repeat the process until the desired number of beetles is transferred. Discard that paper strip and use a fresh one for the next jar you subculture (see note below). Smaller 5" X 3/4" or 5" X 1/2" paper strips are used for subculturing stocks in smaller containers such as square bottles or vials. Strips may be cut even narrower for easier insertion into the smaller containers. Large and small strips can also be "bowed" along the narrow edge with the fingers to allow easier insertion into the vial. A bowed paper strip can actually be more effective for collecting a small number of beetles from a tilted container with a curved surface such as a vial (or corner of a square bottle). (Note: Use one clean strip of paper for each culture jar subcultured. It's best not to lay the strip down on any surface while subculturing a jar because it's possible to unintentionally pick up an unnoticed stray egg, larva or adult from that surface. When in doubt, get a new paper strip!) Use paper transfer whenever possible...it helps prevent transfer of disease by contaminated equipment if disease is a problem, and minimizes the possibility of contamination from a stray egg or small larva left in the sieve or pan. It also selects for the healthiest, most vigorous beetles (with the exception of stocks of beetles with short / defective legs that have difficulty climbing a paper strip (see scoop transfer for these stocks). It's a good idea to spot check each stock subcultured at the time of each subculture. Just place an extra 10 beetles in a petri dish, cool on ice, and inspect the beetles for proper phenotype and state of health with a dissecting microscope (stereoscope). Discard the beetles used for spot check. (If your stock is very small, and every beetle counts, save them, but be very conscientious of good "sterile technique." (i.e., "bang" each petri dish lid and bottom on the tabletop before each use to dislodge any stray eggs or larvae from a previous use.) 5.Scoop or spoon transfer- For Dch3, and other mutants with very short / defective legs, use a small scoop or plastic spoon to collect adults from one jar or bottle and transfer to another. Before use, "sterilize" the scoop or spoon by rapping against the table top several times on both sides. Tilt the bottle so adults move to one side to concentrate them for scooping. Scoop carefully to prevent mashing beetles against the side of the container. Avoid scooping flour as much as possible. (You just want to collect live, healthy adults.) To separate live, healthy adults from flour and dead beetles, you can place the spoonful of beetles in a sorting pan and brush the live beetles into a petri dish before putting them in a jar of new flour. Again, it's a good idea to spot check each stock as you subculture it. Place about 10 adults in a "sterilized" petri dish as mentioned above. 6.Sieving transfer ("Sterile technique")- When paper or scoop transfers are not possible or practical, sieve and select live beetles for subculture by using the following protocol: Bang sieves, receiving pans, and aluminum sorting pans firmly and thoroughly on wastebasket lid immediately before and immediately after use. Bang the plastic transfer funnel lip sharply on the tabletop or wastebasket lid several times. Inspect banged ("sterilized") equipment visually for presence of clinging larvae or adults. If larvae are stuck in the sieve, try to dislodge by additional banging. If this fails, gently poke at them with a brush to encourage them to go on through or withdraw, whichever is the shorter route to getting out. Be careful not to damage them while they are caught in the sieve. If they bleed onto the sieve, their blood and body fluids will corrode the screen. "Squeegee"-sterilize brushes between thumbnail and index finger before using each time. Always sieve into a receiving pan, never onto the table top! Sieve any flour which contains larvae as quickly as possible, with continuous agitation. Dump siftings immediately into sorting pan to minimize the opportunity for larvae to crawl through the screen and get stuck. For those caught in the screen, try to dislodge them first by banging the sieve against the receiving pan (first up-side-down, then right-side-up). Dislodge any remaining larvae by prodding or "tickling" them through the screen gently with a brush. Don't use lateral brushing action to dislodge stuck larvae - rough treatment can squash larvae, and hemolymph from injured larvae can corrode the sieve screen! After sieving diseased stocks, wipe down the sieve and receiving pan with alcohol and dry completely (place on a heat source such as a scope light source, or top of hot incubator, to evaporate excess moisture and solvent). 7.Pan sorting (after sieving): a. Adults- Count or sort the beetles collected in the aluminum pan by brushing adults into a petri dish with a small to medium sized brush. If your sample has a very large number of adults in it, flying beetles can be a problem. (Beetles seem to get more excited and want to fly away when crowded.) You can minimize the problem by first putting all the collected beetles in one or more petri dishes and place lids on the dishes. Then return smaller portions of beetles to the aluminum pan for sorting a bit at a time. b. Pupae- If collecting pupae from a jar with a spoon, you can exclude many adults by tilting the jar to one side. Adults will move to the low side, and you can scoop from the center (Be sure to "sterilize" the spoon first by wiping off and rapping it against the table top several times on both sides!). Sieve, then brush adults and larvae into one petri dish, and brush pupae into another dish. (The collected adults and larvae should be discarded unless the population of that stock is so low that you need to use every beetle to insure survival of the stock.) Note: Sorting adults, pupae, or larvae with a brush is easier if accumulations of exuvia (cast-off skins) are first removed. One method to remove them is by gently blowing them out of the pan, using a side-to-side and near-to-far sweeping motion with your breath, blowing them into a waste basket. It usually takes 3 to four "sweepings" to get most of the exuvia out. (Be careful to blow gently enough that only exuvia, and some dead adults are blown out --not the live adults, pupae and larvae. Dead beetles and exuvia are lighter than live ones and careful blowing helps to separate them.) Another way of separating pupae from adults and larvae is to sift the whole jar, place the adults, larvae and pupae (the siftings), into a petri dish or other clean container, then work with small amounts of the siftings. For each lot, blow off the skins, then shake down the adults and pupae, leaving the larvae. Pour the adults and pupae onto a petri dish lid in a covered sieve receiving pan, and let the adults run off, leaving mostly pupae. Exuvia can also be removed by vacuuming the siftings (from the bottomof the sieve) before placing in the aluminum sorting pan. 8.Use of topping- Topping (coarsely ground wheat) is used to give beetles traction on the flour so they can right themselves if they fall onto their backs (while many beetles in a container can help each other get up, a lone beetle can get stranded on its back and starve to death!). Use topping if: a. Population density is low due to disease or mutation b. Adults have impaired ability to right themselves due to a mutation affecting leg size or shape. For instance, it is wise to use topping with stocks of Dch3* since they can't get around as well as beetles with normal sized and shaped front legs, and since they have lower fertility than other strains. 9.Subculturing schedule- If using a 30? C. incubator temperature, subculture heavily used stocks weekly. Other stocks may be subcultured every other week or monthly. 10.Diseased stocks- Diseased stocks should be subcultured every two days to dilute the disease organisms. Transfer only live beetles! Dead or moribund beetles should be discarded. Eggs may also be collected on Gold Medal flour (or any flour which has been pre sifted through a #50 sieve), and a new stock begun from the debris-free eggs. Allow the adults to lay eggs on the fine flour for 24 hours. Each day, collect the eggs by "double sieving". This method involves using two sizes of sieves, a #25 and a #50, stacked one on top of the other with a receiving pan on the bottom (The #25 is on the top). Adults remain on the #25 sieve and can be placed temporarily in a covered, sterilized petri dish. The eggs will be retained on the #50 sieve, and can then be transferred to a clean glass petri dish. (Alternately, if the two sieves are warped and difficult to separate after sifting, egg collection can be done in two separate siftings: separate the adults from the flour using only the #25 sieve first, then sieve the flour again with the #50 sieve to collect the eggs.) All extraneous material (frass, debris) can then be removed from the collected eggs using a small brush. Put cleaned eggs in jar or bottle of fresh flour for development. (This works for ridding a stock of mites as well as disease). B. TROUBLE SHOOTINGIf a stock is not producing progeny, check the following:
Parasitic mites tend to hang all over the adults, sometimes to the point of giving them a frosted look, and also hide under the wings and elytra. They seem to prefer female beetles, possibly as a way of being near eggs which they may feed on. A permanent or long term cure is a lot of work initially. A subset of adults need to be cleaned. This means putting them on ice and removing the mites with a vacuum probe or aspirator. When they are mobile and dry, put them in fine flour with topping for egg collection. Collect the eggs 1-3 days later (depending on the number of adults ovipositing). Now come the hardest part. Put the eggs in a glasspetri dish on some dark paper or other good-contrast surface, under the microscope. With an insect pin and a small vial of ethanol, remove EVERYTHING that is not a plump, healthy egg. Dip the head of the pin in the ethanol, and then blot up the trash. Swish or shake the dish and repeat. You have to be careful of mites that are feeding on the eggs, as they swell up almost egg-size, and the egg wraps around them as it is depleted. Put the now "sterile" (mite free) eggs in new flour and hope for the best. Freeze out your equipment for 24 hours. C. TROUBLE PREVENTION
D. Disease & MitesEggs may also be collected on Gold Medal flour (or other equally fine flour) , and a new stock begun from the debris-free eggs. Allow the adults to lay eggs on the fine flour for 24 hour periods of time. Each day, collect the eggs by double sieving. This method involves using two sizes of sieves, a #25 and a #50, stacked one on top of the other . The #25 is placed on top, with the #50 between the #25 and the receiving pan. Adults remain on the #25 sieve and can be placed temporarily in a covered, sterilized petri dish. The eggs will be retained on the #50 sieve, and can then be transferred to a clean petri dish. (Alternately, if the two sieves being used are warped and difficult to separate after sifting, egg collection can be done in two separate siftings: separate the adults from the flour using only the #25 sieve first, then sieve out the eggs using only the #50 sieve.) All extraneous material (frass, debris) can then be removed from the collected eggs using a small brush. Put cleaned eggs in jar or bottle of fresh flour for development. This works for ridding a stock of mitesas well as disease. Parasitic mitescan easily retard or destroy an otherwise healthy stock. The mites hang all over the adults, sometimes to the point of giving them a frosted look. They seem to prefer females. A permanent or long term cure can be achieved, with a lot of work.
E. Developmental Rates of Tribolium castaneum
(The reproductive lifetime is 3-4 months for females and 4-6 months for males. Isolated males have been known to live for up to a year) Note: At 22?C, development is much slower. F. Sexing TriboliumSeparating the sexes is necessary in order to run a number of genetics tests. Both adults and pupae can be sexed. If the intended cross must be a virgin cross, it is necessary to sex the beetles as pupae to insure no previous mating has taken place. Following are some materials and methods which have worked well in our laboratory, plus suggested alternate materials you might use.
1. Pupae(Sexing beetles as pupae rather than as adults is easiest since pupae move very little compared to the adults, and do not need to be immobilized by cooling them on ice.) a. Materials (Microscope, light source, working surface, manipulating tools) Microscope : A stereoscope is needed to sex the pupae. You will want to be able to magnify the pupae by at least 20 - 30X. A zoom lens stereoscope is very handy. Light source : A good light source will reduce eyestrain if you are going to be looking at many pupae. The best is a fiber optics light system. It is both a cooler light source than conventional lights, and those on gooseneck pipes can be aimed at exactly the area you need to focus on. We use a fiber optics light with two light pipes. If you use a standard light, be careful not to overheat your pupae by having the light source too close to them. Working surface : A small plate of a non-static generating material (approx. 3" x 4") is very handy for separating the sexes. We use a 3" x 4" piece of styrofoam backed posterboard. This has a thickness of about 1/4" which makes it easy to pick up, is light weight, and has a smooth surface to work on. We have chosen a deep blue color, since that color provides a good contrast to the color of the pupae. Any dark color will do. Light colors should be avoided because they cause a glare from the lights. Manipulating tools : A small natural bristle brush can be used to move the pupae on the "plate". Alternately, a commercial or a homemade vacuum probe can be used to manipulate the pupae. We use a version available through the Jensen Tool catalog, which is hooked up to the vacuum system in our building. (The same probe could also be connected to small electrical vacuum pump) . A much simpler version can be made from a plastic drinking straw, a 2-foot piece of flexible rubber tubing (approx. 1/8" internal diameter) , and a plastic pipette tip. In this case, the vacuum is supplied by the user's mouth. Other : Plastic petri dishes or other small containers can be used to temporarily hold the pupae both before and after sexing. These same containers, or small bottles or vials which contain about 1"of flour can be used to hold the pupae until they eclose to adulthood. Any container used for this purpose should have a lid which would keep the wandering adults from escaping. (The lid also needs to have small air holes placed in the top if it is a very tight fitting lid. Petri dish lids do not need air holes.) A plastic funnelis handy for pouring pupae or adults from a sorting pan into a bottle or jar.
a. Materials (ice bucket, ice block) Ice bucket: Any container which can hold crushed ice can be used to precool adult beetles. The small styrofoam boxes or buckets which are used for picnics or fishing are perfect for this. . Ice block: This is used to keep the beetles immobile while you are looking at them under the microscope. We use small, flat plastic tissue culture bottles which we fill with water about 1/4"at a time until they are mostly full. (Don't fill any container completely full because it will crack or burst when frozen!) Any low-profile container which can hold crushed ice could be used; for instance, a large petri dish full of crushed ice. The pre-cooled beetles are then placed in a smaller, low-profile container (such as a smaller petri dish lid) , and this smaller container of beetles is placed on the larger low-profile container filled with ice. You should be able to place this assemblage under your stereoscope. The beetles should remain immobile long enough for you to be able to sex them. (Tip: The ice eventually melts, allowing the beetles to "wake up", so you will want to limit the number of beetles you sex at onetime to a number compatible with the "staying power" of your cooling equipment.) .
|
||||||||||||||||||
| * Indicates link to external site |


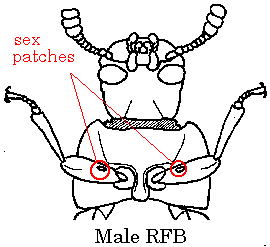 Line up the adults as with the pupae above, and separate them into two new lines according to sex. Use the diagram at right to distinguish between males and females. The males have a small patch of short bristles on the inside of the first pair of legs, about 1/3 the distance out from the bases. If the patches have picked up flour, they will appear like two domes of flour or flour paste, and will be fairly easy to see. If they have not yet picked up flour, they will appear as slightly darker, textured spots on the legs. (Changing the angle of light or changing the position of the beetle can help make the patches more visible if you are having trouble seeing them.)
Line up the adults as with the pupae above, and separate them into two new lines according to sex. Use the diagram at right to distinguish between males and females. The males have a small patch of short bristles on the inside of the first pair of legs, about 1/3 the distance out from the bases. If the patches have picked up flour, they will appear like two domes of flour or flour paste, and will be fairly easy to see. If they have not yet picked up flour, they will appear as slightly darker, textured spots on the legs. (Changing the angle of light or changing the position of the beetle can help make the patches more visible if you are having trouble seeing them.)