| More Information about Pyraloidea |

|
The Pyraloidea is estimated to be the second largest superfamily in the Lepidoptera, with more than 16,000 described species worldwide. Pyraloid caterpillars are very diverse in what they eat: they consume dried or decaying plant or animal matter, wax in bee and wasp nests, and living plants. Some are known to be inquilines in ant nests (some Galleriinae), predators of scale insects (some Phycitinae), and aquatic scavengers in flowing water (some Nymphulinae) (Solis 1997). The plant feeders can be leaf rollers, leaf tiers, leafminers, and stem borers, and sometimes a combination. Pyraloid caterpillars are pests that cause damage and economically affect crops such as rice, sugarcane, corn, tomato, and many more; some are worldwide pests of stored products such as grains and fruits (Solis 1996).
Because so many pyraloid caterpillars are intercepted at ports in commodities being imported into the United States, the Pyraloidea part of "Keys for the identification of some lepidopterous larvae frequently intercepted at quarantine" by Hahn W. Capps, Division of Insect Identification, Bureau of Entomology and Plant Quarantine, U. S. Department of Agriculture was first published in 1939. It was published again in Spanish (Capps 1955) by the Agriculture Department of Mexico and again in English (Capps 1956, 1963) with only nomenclatural revision. It was not significantly revised again until 1986, when D. M. Weisman published "Keys for the identification of some frequently intercepted lepidopterous larvae." He added 40 species and replaced the Heinrich (1916) system of setal nomenclature with the Hinton (1946) system. The revision presented here adds new taxa, incorporates recent new combinations, and provides keys to the family and subfamily levels of Pyraloidea. This revision also updates distributions, stating if taxa occur in restricted areas of the continental U.S. and Hawaii. A "Note" section explains changes and additions, adds relevant information, and gives references to further information. Two tables provide host and classification information.
The Pyraloidea has undergone both phylogenetic and nomenclatural changes because it is a group where taxonomists are actively pursuing questions that have both theoretical and applied ramifications. In the 1980's, Minet published a series of morphological papers on the tympanal organs in the Lepidoptera, including the Pyraloidea (1982). Based on the morphologically distinct tympanal organs and the work on larvae by Hasenfuss (1960), Minet proposed elevating two groups, known in the informal sense as Pyraliformes and Crambiformes (Munroe 1972), to Pyralidae and Crambidae. Most workers in the Pyraloidea agree with Minet (e.g., Munroe 1989; Solis & Mitter 1992, Solis & Maes 2002). Taxonomy is not a static field but a field where new morphological and biological information continually becomes available, and it is necessary to change the classification to reflect this new information. In addition, several major checklists (Munroe et al. 1995; Shaffer et al. 1996) from several major geographic areas have been published in the last ten years with many new combinations and synonymies. Table 1 gives the current classification of Pyraloidea as an alphabetical list of the taxa treated in this work in the two families by subfamily, with the number of the couplet where they are found in the key for quick retrieval.
DESCRIPTION OF THE KEY AND ITS COMPONENTS
Capps' (1939) description of the function and basis of his key is still applicable today: "The following keys are intended to assist quarantine inspectors in recognizing the lepidopterous larvae most frequently intercepted at ports of entry and are based on the differential characters noted in the literature, and on the larval collection and host catalogue in the United States National Museum." The title of this revision reflects a change from "most frequently" taxa intercepted to "selected" taxa intercepted. I retained all taxa included in Weisman's key even though the species may no longer be intercepted frequently; this in part because the species intercepted depend on the commodities being imported into the U.S. and these species may again be intercepted in the future. The addition of species to this current key is based on the actual interceptions submitted by APHIS port identifiers. Specimens are submitted for identification until the port identifier receives "port authority" for the identification of particular species; and then they no longer send specimens for verification of that species. The top twelve species sent into the SEL (Systematic Entomology Laboratory) for identification in order from more frequent to less frequent during 1998 were: Ectomyelois ceratoniae, Cadra cautella, Leucinodes orbonalis, Diatraea considerata, Spoladea recurvalis, Neoleucinodes elegantalis, Etiella zinckenella, Conogethes sp., Pyrausta sp., Phidotricha erigens, Plodia interpunctella.
Capps (1939) also wrote: "In using the keys, it should be borne in mind that their validity is dependent on three factors, viz., (1) structure, (2) origin, and (3) host." The origin referred to by Capps indicates the country where the commodity supposedly originated and does not imply evolutionary origin; for this reason Weisman (1986) probably chose to use the term "distribution" rather than "origin." The origin documented by port identifiers is the origin of the vehicle transporting the commodity prior to entering the U.S. The point of origin of the insect could be several ports removed if the vehicle made multiple stops, or entirely outside the vehicle's itinerary if infested cargo was transferred en route.
Further, Capps (1939) wrote: "Moreover, the characters used for separating the families are not completely diagnostic for the entire family but will serve to separate the species treated here." This is emphasized for two reasons: one, the percentage of lepidopterous larvae known is very small, usually only the larval morphology of the pest species in a genus is well known, and hence, the distribution of the characters across taxa are unknown; and two, the loss or reduction of characters in larvae in general is inferred to occur extensively (see also Passoa 1985).
All current taxonomic and phylogenetic information has been incorporated into the revision of this key. Distributions vary according to the information provided with the submitted material and are based specifically on the usage by port identifiers, for example, a country versus an area of a continent. It is stated if the species occurs in Hawaii or a few states in the continental U. S. Changes in distribution in this revision are based on the current literature and unpublished localities in the Pyraloidea collection of the National Museum of Natural History, Smithsonian Institution, Washington, D.C. (USNM). New records in the U.S. are taken into account if there is evidence to support that a population has been established. It is common in certain parts of the U.S. adjoining the Gulf of Mexico to catch one or more adult(s) of a species at light, but this is not evidence that the species is established in the U.S. Specifically, distribution records from Hawaii are from Nishida (1992); it uses three words to reflect residency status: endemic, indigenous, and adventive. I used only adventive when applicable: "immigrant" ; used in place of "introduced" to differentiate from those that were purposely introduced. Species that are known only from quarantine records (reported as intercepted) or those considered not established are present in the database, but do not appear in the checklist" (Nishida 1992). The "Old World" includes all land masses except the Western Hemisphere.
The plant names are based primarily on the names given to commodities being imported or brought into the U.S. for any variety of purposes; in this work the biological term "host" and the economic term "commodity" are often one and the same. The names of hosts are either a scientific name or a common name as supplied by port identifiers and checked against Brako, Rossman, and Farr (1995) for U.S. names, and Mabberley (1997) and the Missouri Botanical Garden's VAST nomenclatural database for all other localities and are listed under the "Hosts" section of each species. In the key, the 2006 host records are directly from the SELIS database (Systematic Entomology Laboratory Identification Service) as submitted by port identifiers and listed alphabetically. Pre-1998 records can be from a variety of sources and are primarily those listed in Weisman (1986), with additions from the SELIS database, the USNM larval collection, and are mainly historical records. If the scientific name of a host appeared in both the 2006 list and pre-1998 list, it was removed from the pre-1998 list. The lists of hosts at times lack detail (e.g. "stored vegetable products" ) because many pyraloid pest species are highly polyphagous. Table 2 gives the hosts of the pyraloid larvae. If a scientific name for the commodity is given, the table refers to the common name as given by the port identifiers also; scientific names were not generally used prior to the mid-1980's. The common name is followed by the scientific name in brackets for purposes of cross-indexing.
The "Note" sections comment on a variety of topics that may be useful to the port identifier, it is not meant to be comprehensive: on character variability, explanations of recent nomenclatural changes, nomenclatural method of reporting
based on morphological and distributional information available, and relevant literature. The amount of literature available is scattered and very large for pest species, and is less large for geographical works (e.g. Carter 1984; Mutuura et al. 1965). This work does not attempt to review the entirety of the literature, but rather to point to seminal literature that provides relevant information.
HOW TO DISTINGUISH PYRALOIDEA LARVAE
Pyraloidea larvae can be distinguished from other Lepidoptera larvae by a combination of characters. Many "micro" lepidopteran groups have 3 setae in the prespiracular group of the prothorax (Fig. 1), but some may have 2 or 1 (Stehr 1987) and they do not have typical pyraloid crochets (see below). Pyraloids, noctuids, and other "macro" lepidopteran groups have two setae in the prespiracular group of the prothorax (Fig. 2) (Stehr 1987). The Noctuoidea and Carposinidae, two groups that are intercepted frequently and are of importance to port indentifiers, can be confused with pyraloids based on the presence of two setae in the prothoracic prespiracular group. But pyraloids can be distinguished from noctuoids because noctuoids have the crochets in a mesoseries (Fig. 3), and pyraloids have the crochets in a complete circle or penellipse (Figs. 4-5).
Larvae of the Carposinidae are also confused with pyraloids because they also have two setae in the prespiracular group of the prothorax and crochets in a complete circle. Generally, pyraloids can be separated from carposinids because pyraloids have 3 subventral setae on abdominal segments 3 to 6 (Fig. 6), and carposinids usually have 4 subventral setae (Fig. 7), but the number of subventral setae may vary from segment to segment (see Common 1990). It should be noted here that Weisman (1986) used "the spiracle on abdominal segment 8 well above level of those on preceding segments" to separate them from pyraloids, but many pyraloids have the spiracle on segment 8 above the level of those on the preceding segments.
For recent, more general information on other nearctic pyraloid larvae and lepidopteran larvae and comparisons to other families and other geographic regions see Stehr (1987) and Common (1990).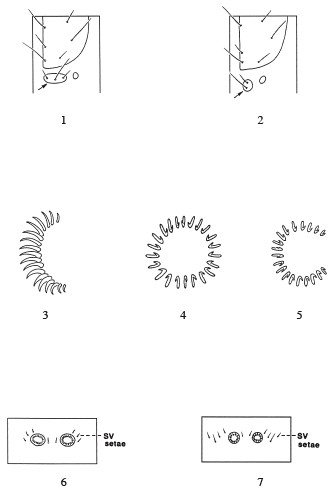
Table 1. Classification of Included Pyraloidea (number refers to couplet in the key).
| CRAMBIDAE - 27 CRAMBINAE Chilo suppressalis (Walker) - 31 Diatraea sp. - 31 Eoreuma loftini (Dyar) - 30 EVERGESTINAE Evergestis rimosalis (Guenee) - 37 GLAPHYRIINAE Hellula rogatalis (Hulst) - 39 Hellula phidilealis (Walker) - 39 ACENTROPINAE Parapoynx diminutalis Snellen - 27 PYRAUSTINAE Achyra rantalis (Guenee) - 41 Ostrinia nubilalis (Huebner) - 36 Pyrausta sp. - 33 SPILOMELINAE Conogethes spp. - 34 Diaphania nitidalis (Cramer) - 50 Diaphania indica complex - 50 Duponchelia fovealis Zeller - 48 Hendecasis duplifascialis Hampson - 48 Herpetogramma bipunctalis (Fabricius) - 43 Leucinodes orbonalis (Guenee) - 51 Lineodes integra (Zeller) - 46 Loxomorpha flavidissimalis Grote - 41 Maruca vitrata (Fabricius) - 35 Megastes sp. - 35 Neoleucinodes elegantalis (Guenee) - 51 Rhectocraspeda periusalis (Walker) - 43 Spoladea recurvalis Fabricius - 45 Udea rubigalis (Guenee) - 46 SCHOENOBIINAE - 28 | PYRALIDAE - 2 CHRYSAUGINAE - 22 EPIPASCHIINAE Phidotricha erigens (Ragonot) - 19 GALLERIINAE Alpheias conspirata Heinrich - 24 Corcyra cephalonica (Stainton) - 26 Paralipsa gularis (Zeller) - 26 Genopaschia protomis Dyar - 24 Trachylepidia fructicassiella Ragonot - 25 PHYCITINAE Amyelois transitella (Walker) - 13 Ancylostomia stercorea (Zeller) - 8 Cadra cautella (Walker) - 17 Cadra figulilella (Gregson) - 18 Cadra calidella (Guenee) - 18 Cryptoblabes sp. - 6 Ectomyelois ceratoniae (Zeller) - 13 Elasmopalpus lignosellus (Zeller) - 6 Ephestia elutella (Huebner) - 16 Ephestia kuehniella (Zeller) - 16 Etiella zinckenella (Treitschke) - 20 Fundella pellucens Zeller - 10 Homoeosoma electellum Hulst - 11 Hypsipyla sp. - 9 Moodna bisinuella Hampson - 9 Mussidia nigrivenella Ragonot - 4 Plodia interpunctella (Huebner) - 14 PYRALINAE Pyralis farinalis Linnaeus - 21 Aglossa caprealis (Huebner) - 21 |
