| Page 3 |

|
|
1 - Ventilation Equipments
2 - Page 2 3 - Page 3 4 - Page 4 |

 HOME
HOME |
![]() Biological Safety Cabinet in Quicktime Movie
Biological Safety Cabinet in Quicktime Movie
RunningTime: 20 minutes
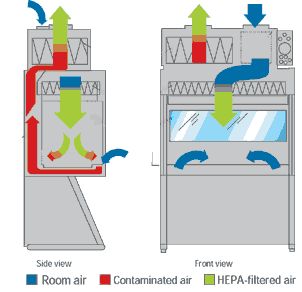 |
The Class I Biological Safety Cabinet(top right) provides personnel and environmental protection, but no product protection.
Unfiltered room air is drawn across the work surface and up the exhaust system through the HEPA filters. The Cabinet is hard-ducted to the building exhaust system, and the building exhaust fan provides the negative pressure necessary to draw room air into the cabinet.
 |
The Class II Biological Safety Cabinet (bottom right) can be found in almost every tissue culture lab. It is designed to:
- Protect you from being exposed to infectious aersoles that may be generated within the cabinet.
- Protect your cultures from microbial contaminants that are ubiquitous in room air
Your cultures ar protected by the cabinet downard particular air (HEPA) filters. HEPA filters also treat cabinet exhaust air.
 |
The rapid movement of a worker's arms in a sweeping motion into and out of the cabinet will disrupt the air curtain and may compromise the partial barrier containment provided by the BSC. Moving arms in and out slowly, perpendicular to the face opening of the cabinet, will reduce this risk.
Other personnel activities in the room (e.g., rapid movement, open/closing room doors, etc.) may also disrupt the cabinet air barrier .
Manipulation of materials should be delayed for approximately one minute after placing the hands/arms inside the cabinet. This allows the cabinet to stabilize and to "air sweep" the hands and arms to remove surface microbial contaminants. When the user's arms rest flatly across the front grille, room air may flow directly into the work area, rather than being drawn through the front grille. Raising the arms slightly will alleviate this problem.
The front grille must not be blocked with research notes, discarded plastic wrappers, pipetting devices, etc. All operations should be performed at least four "4" inches from the front grille on the work surface.
Cabinet blowers should be operated at least three to five minutes before beginning work to allow the cabinet to " purge". This purge will remove any particulates in the cabinet.
The work surface, the interior walls (not including the supply filter diffuser), and the interior surface of the window should be wiped with 70% ethanol (EtOH), a 1:100 dilution of household bleach (i.e., 0.05% sodium hypochlorite), or other disinfectant as determined by the investigator to meet the requirements of the particular activity. When bleach is used, a second wiping with sterile water is needed to remove the residual chlorine, which may eventually corrode stainless steel surfaces. Wiping with non-sterile water may recontaminate cabinet surfaces, a critical issue when sterility is essential (e.g., maintenance of cell cultures).
Similarly, the surfaces of all materials and containers placed into the cabinet should be wiped with 70% ETOH to reduce the introduction of contaminants to the cabinet environment. This simple step will reduce introduction of mold spores and thereby minimize contamination of cultures. Further reduction of microbial load on materials to be placed or used in BSCs may be achieved by periodic decontamination of incubators and refrigerators.
Material Placement Inside the BSC
Plastic-backed absorbent toweling can be placed on the work surface (but not on the front or rear grille openings). This toweling facilitates routine cleanup and reduces splatter and aerosol formation during an overt spill. lt then can be folded and placed in an autoclavable biohazard bag when work is completed.
A typical layout for working "clean to dirty" within a Class II BSC. Clean cultures (left) can be inoculated (center); contaminated pipettes can be discarded in the shallow pan and other contaminated materials can be placed in the biohazard bag (right). This arrangement is reversed for left-handed persons 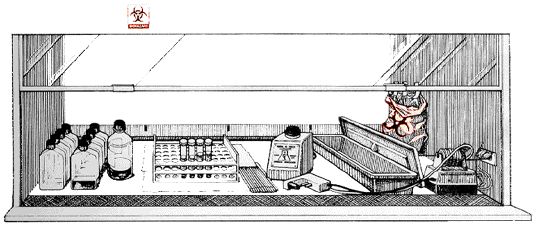
Certain common practices interfere with the operation of the BSC.
- The autoclavable biohazard collection bag should not be taped to the outside of the cabinet.
- Upright pipette collection containers should not be used in BSCs nor placed on the floor outside the cabinet.
- Only horizontal pipette discard trays containing an appropriate chemical disinfectant should be used within the cabinet.
- Potentially contaminated materials should not be brought out of the cabinet until they have been surface decontaminated. Alternatively, contaminated materials can be placed into a closable container for transfer to an incubator, autoclave or for other decontamination treatment.
Laboratory Hazards
Class II cabinets are designed so that horizontally nebulized spores will be captured by the downward flowing cabinet air within fourteen inches of travel. Therefore, as a general rule of thumb, keeping clean materials at least one foot away from aerosol-generating activities will minimize the potential for cross-contamination.
Several measures can be taken to reduce the chance for cross-contamination when working in a BSC.
- Opened tubes or bottles should not be held in a vertical position.
- Investigators working with Petri dishes and tissue culture plates should hold the lid above the open sterile surface to minimize direct impaction of downward air.
- Bottle or tube caps should not be placed on the toweling.
- Items should be recapped or covered as soon as possible.
An open flame creates turbulence which disrupts the pattern of air supplied to the work surface. When deemed absolutelv necessary, touch-plate microburners equipped with a pilot light to provide a flame on demand mav be used. Internal cabinet air disturbance and heat buildup will be minimized. The burner must be turned off when work is completed. Small electric "furnaces" are available for decontaminating bacteriological loops and needles and are preferable to an open flame inside the BSC. Disposable sterile loops can also be used.
Investigators must determine the appropriate method of decontaminating materials that will be removed from the BSC at the conclusion of the work. When chemical means are appropriate, suitable liquid disinfectant should be placed into the discard pan before work begins. Items should be introduced into the pan with minimum splatter, and allowed appropriate contact time as per manufacturer's instructions. Alternatively, liquids can be autoclaved prior to disposal. Contaminated items should be placed into a biohazard bag or discard tray inside the BSC. Water should be added to the bag or tray prior to autoclaving.
When a steam autoclave is to be used, contaminated materials should be placed into a biohazard bag or discard pan containing enough water to ensure steam generation during the autoclave cycle. The bag should be taped shut or the discard pan should be covered in the BSC prior to removal to the autoclave. The bag should be transported and autoclaved in a leakproof tray or pan.
Surface Decontaminiation
All containers and equipment should be surface decontaminated and removed from the cabinet when work is completed. At the end of the work day, the final surface decontamination of the cabinet should include a wipe-down of the work surface, the cabinet's sides and back, and the interior of the glass. If necessary, the cabinet should also be monitored for radioactivity and decontaminated when necessary. Investigators should remove their gloves and gowns and wash their hands as the final step in safe microbiological practices.
Spills
Small spills within the BSC can be handled immediately by removing the contaminated absorbent paper toweling and placing it into the biohazard bag. Any splatter onto items within the cabinet, as well as the cabinet interior, should be immediately wiped with a towel dampened with decontaminating solution. Gloves should be changed after the work surface is decontaminated and before placing clean absorbent toweling in the cabinet. Hands should be washed whenever gloves are changed or removed.
Spills large enough to result in liquids flowing through the front or rear grilles require more extensive decontamination. All items within the cabinet should be surface decontaminated and removed. After ensuring that the drain valve is closed, decontaminating solution can be poured onto the work surface and through the grille(s) into the drain pan.
Twenty to thirty minutes is generally considered an appropriate contact time for decontamination, but this varies with the disinfectant and the microbiological agent. Manufacturer's directions should be followed. The spilled fluid and disinfectant solution on the work surface should be absorbed with paper towels and discarded into a biohazard bag. The drain pan should be emptied into a collection vessel containing disinfectant. A flexible tube should be attached to the drain valve and be of sufficient length to allow the open end to be submerged in the disinfectant within the collection vessel. This procedure serves to minimize aerosol generation. The drain pan should be flushed with water and the drain tube removed.
| << Previous 1 2 [3] 4 Next >> |
