| Station Note 01-94: Polyacrylamide (PAM) - A New Weapon in the Fight Against Irrigation-induced Erosion |

|
USDA-ARS Northwest Irrigation & Soils Research Lab Station Note #01-94 (Revised)
POLYACRYLAMIDE (PAM)
A New Weapon in the Fight AgainstIrrigation-induced ErosionR.E. Sojka and R.D. LentzUSDA-Agricultural Research Service3793 N 3600 E, Kimberly, ID 83341 PH:(208)423-6531, FAX 6555
Since the late 1980's there has been renewed interest in the use of water soluble polymers for soil physical improvement. Several factors have contributed to this, especially use of new, more efficient application strategies and availability of new, inexpensive, more effective polymers. The most striking results have been with the use of high-potency flocculents in surface irrigation water for erosion and infiltration management. The technology has received extensive coverage in the national media. This has generated intense researcher and farmer interest. Some regard PAM effects as exotic and see it as a "miracle cure" to several of irrigated agriculture's most persistent soil management problems. PAM can provide striking results and holds great promise. An effort is needed, however, to increase the overall understanding of what PAM is, how it works, and how it can impact irrigated soil and water management practices. PAM is widely used as a settling agent for food processing and packaging, paper production, mine and municipal waste water treatment, as a clarifier for sugar extraction and potable water treatment.
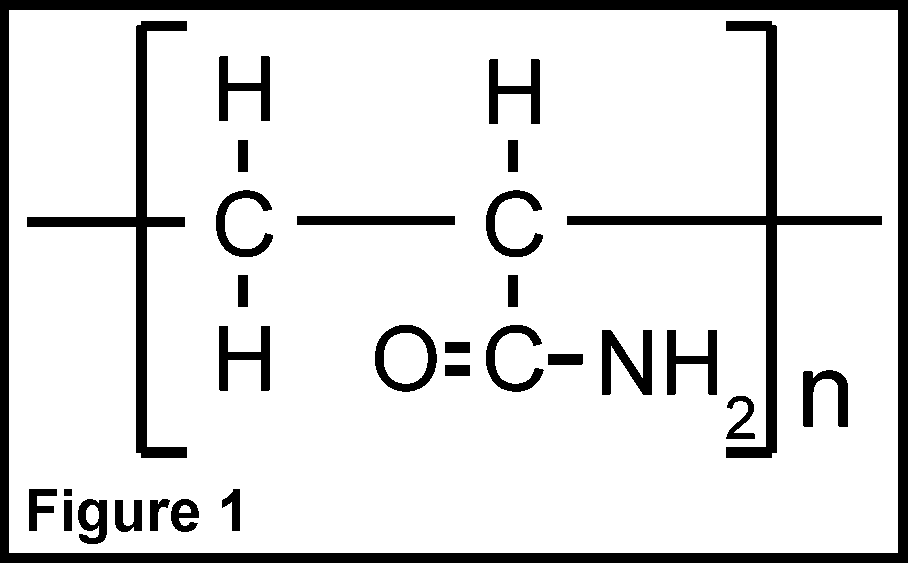 What is PAM? PAM is an acronym for the chemical polyacrylamide. As the prefix "poly" implies, it is a chemical made up of 'many' subunits of "acrylamide" molecules. Acrylamide is a relatively simple organic compound that can be linked to identical copies of itself to form chain-like molecules. A single acrylamide molecule is referred to as the "monomer", while chained monomers (Fig. 1) are the "polymer". PAM is commonly synthesized from natural gas.
What is PAM? PAM is an acronym for the chemical polyacrylamide. As the prefix "poly" implies, it is a chemical made up of 'many' subunits of "acrylamide" molecules. Acrylamide is a relatively simple organic compound that can be linked to identical copies of itself to form chain-like molecules. A single acrylamide molecule is referred to as the "monomer", while chained monomers (Fig. 1) are the "polymer". PAM is commonly synthesized from natural gas.
Thousands of natural and man-made organic polymer compounds exist. Glucose, the simplest sugar, is a monomer. Starch and cellulose are composed of long chains of glucose monomer. Chemical and physical characteristics of the monomer and polymers are typically very different. For example, glucose sugar is easily broken down, whereas cellulose is resistant. Starch, an intermediate polymer, breaks down less easily than glucose, but easier than cellulose.
Similarly, the chemical and physical properties of PAM differ greatly from the acrylamide monomer and also vary with the length of the polymer chain. PAM can be synthesized to have various chain lengths. There are, in fact, thousands of "kinds" of PAM. The term PAM is generic. Depending on chain length and minor alterations of some of the subunits, PAMs can have unique properties. The PAMs of current interest to agriculture typically have more than 100,000 monomer units per molecule. The PAMs used in irrigation for erosion control are copolymers. In about one fifth of the linked subunits, the -NH2 group is replaced by a different functional group that gives the dissolved polymer molecule a moderate negative charge (anionic).
Is PAM new to agriculture? Yes and no. Although PAM has been used for soil structure improvement since the 1940's, and in agriculture since the 1950's the kinds of PAM used and methods of application were different. Early PAM's had lower molecular weights than today's PAMs. They were applied to soil at high rates >450 lb/acre (>500 kg/ha), and were incorporated into the top soil by tillage. The idea was to stabilize the structure of the plow-layer.
The new generation of agricultural PAMs have ultra high molecular weights and are moderately (18%) anionic. They are applied via irrigation water to only that small part of the soil that plays a role in the physical processes of erosion, sealing and crust formation (i.e. the surface few millimeters of soil depth in the treated zone). In furrow irrigation this has produced dramatic erosion reduction and infiltration stabilization with very small PAM applications per irrigation, 1 lb/acre (about 1 kg/ha).
What does PAM do? Water-applied PAM increases soil cohesion and strengthens the aggregates it contacts in the furrow by binding exposed soil particles together more securely. This greatly reduces detachment and transport of sediments in irrigation runoff. Soil erodibility at the soil water interface is reduced by improved inter-aggregate bonding and better maintenance of surface roughness. PAM also acts as a settling agent. It flocculates (clumps together) the fine particles dispersed by and carried in the flow, causing them to settle to the furrow bottom. Fewer dispersed fine particles remain in the infiltrating water to block pores and reduce infiltration rates. Pore structure is maintained, preventing the usual infiltration rate reduction. This decreases runoff rate and amount, which further reduces stream force, carrying capacity, and transport volume. Additional factors that may contribute to reduced erosivity of PAM-treated irrigation water may include induction of laminar flow, changes in viscosity and/or surface tension of water near the soil-water interface. The combination of all these effects has reduced soil loss up to 99%, and typically around 90-95%.
How is PAM applied? Erosion control has been achieved with 10 ppm PAM in the advancing furrow stream. Once the advance is complete, however, PAM need not be added to the balance of an irrigation. Thus, when expressing PAM use for the total water applied in a single irrigation, the net application is typically about 1 lb/acre-inch of water applied. The concentration required can vary somewhat for specific soil properties, field conditions, or irrigation parameters. Injection of PAM into furrow streams should stop shortly after runoff begins. PAM treatment has usually been by injection of small amounts of concentrated stock solutions into the irrigation water supply. There is some indication that direct powder addition may be feasible, but the concept has not been extensively tested. The 10 ppm furrow advance treatment should be applied for the first irrigation of the season, and whenever soil in the furrow has been disturbed by cultivation or traffic. Without any retreatment, protection declines about 50% for each subsequent irrigation so additional treatments may be warranted. This means that typical seasonal application totals will range from 3-7 lbs per acre.
Can there be problems applying PAM? Yes. Currently PAMs are most commonly produced as dry granules. They completely dissolve and remain dissolved if mixed properly (slow addition to water under strong sustained agitation). If added too quickly or if not vigorously stirred the granules rapidly form non-dissolvable gels on contact with water or collect in low turbulence areas as syrupy concentrates that dissolve slowly in an uncontrolled pattern over a period of hours or days. Furthermore, because PAM is an ultra potent flocculent, it causes rapid settling of any sediments already suspended in irrigation water. Thus, where sediment content of the irrigation water is high, the resulting siltation in the water delivery system may cause problems. This engineering hurdle is the focus of current application research.
Can PAM be sprinkler-applied or sprayed on? Yes, but much higher rates are needed for results comparable to those seen with furrow irrigation. Furrow irrigation only treats about 25-30% of the field surface area (the wetted perimeter). Sprinkler irrigation treats 100%. Sprinkler irrigation has an additional energy component causing soil detachment and transport--water drop impact. Water drops also excavate and mix a few millimeters of soil in the erosion process. These combined factors greatly increase the amount of PAM needed to stabilize surface structure. PAM has also been found less effective when sprayed on in a separate application step compared with irrigation-borne application.
Will PAM require irrigation management changes if I use it? Yes. PAM increases infiltration rate, furrow advance time, and lateral wetting compared to comparable application rates of non-amended water. If an irrigation is not adjusted, over-watering of the upper end and/or underwatering of the lower ends could be worsened. Fortunately, PAM's erosion preventing properties will usually allow farmers to use higher water application rates in order to push water more quickly across the field while still minimizing soil loss. This modification is expected to improve water application uniformity, reducing both upper-end leaching and lower-end water stress. Improved lateral wetting has been documented. This may reduce irrigation duration needed for germinating or seedling crops.
Are there safety or environmental concerns with PAM? Yes and no. When spilled on hard surfaces, PAM solutions are extremely slippery and hazardous to foot and vehicle traffic. PAM dust is highly hygroscopic and, if inhaled, could impair breathing. The chemical safety of PAM per se depends largely on three factors: 1) the specific chemical configuration of the polyacrylamide molecule, 2) the degree and nature of non-polyacrylamide contamination in the manufacturing process, and 3) the nature of PAM's decomposition products. Certain neutral and cationic PAMs at very high exposure levels produce irritation in humans and are somewhat toxic to certain aquatic organisms. The particular anionic PAM we have used does not demonstrate such toxicities, even at high exposure rates. The manufacturing contaminant of greatest concern is the acrylamide monomer. It is toxic. It is, however, kept below strict limits (0.05%) in industrial synthesis. At these levels this PAM is recognized by both EPA and FDA as safe for sensitive environmental and food production uses below set concentration limits. Concerns sometimes expressed regarding PAM toxicity and/or monomer contamination often originate from other polyacrylamide species (other PAMs) or from less rigidly controlled (incomplete) synthesis processes (e.g. low molecular weight PAM synthesis for electrophoresis). PAM decomposition does not appear to result in release of acrylamide monomer, and what little monomer is applied as a contaminant has been shown highly susceptible to decomposition in a normal surface soil environment.
Where can I get PAM and what does it cost? PAM is manufactured by numerous chemical companies. At this writing, we are aware of two manufactured forms of PAM that have labels for use as a soil amendment in some states. These are produced by Cytec Industries Inc. and Allied Colloids Inc. The costs for PAM at the point of delivery to farmers is not known at this time. PAM is a relatively inexpensive polymer delivered in bulk at point of use in industrial settings, but mass-distribution to rural users will probably increase its cost. Ball park estimates of a $3-5 per pound cost to farmers are probably not unreasonable. PAM should be used in strict compliance with state and federal label requirements.
General Reading
De Boodt, M.F., M.H.B. Hayes and A. Herbillon (eds). 1990. "Soil Colloids and Their Associations in Aggregates." NATO ASI Series. Series B: Physics Vol. 215. 598 pages. Plenum Press, New York.
Moldenhauer, W.C. (ed). 1975. "Soil Conditioners" 186 pages, No. 7 in the SSSA Special series. Soil Sci. Soc. of Am., Madison, WI.
Soil Science, Vol.141 No. 5. May 1986. Lowell A. Douglas (ed). A series of papers on the topic of soil conditioners and plant growth.
Soil Science, Vol. 158 No. 4. Oct. 1994. Robert L. Tate (ed). A series of papers on polyacrylamide use in irrigation water.
Acrylamide and PAM Toxicity/Decomposition
Soil Science, Vol. 158 No. 4. Oct. 1994. Robert L. Tate (ed). A series of papers on polyacrylamide use in irrigation water.
Abdelmagid, H.M., and M.A. Tabatabi. 1982. Decomposition of acrylamide in soils. J. Env. Qual. Vol. 11, No. 4. 701-704
Lande, S.S., S.J. Bosch and P.H. Howard. 1979. Degradation and leaching of acrylamide in soil. J. Env. Qual. Vol.8, No. 1. 133-137.
Wilson, A.D., and S. Crisp. 1975. Rigid, highly carboxylated ionic polymers.p.208-257. In L. Holliday (ed.) Ionic Polymers. Chapman and Hall, New York.
Thomas, W.M. 1964. Acrylamide polymers. Encycl. Polymer Sci. Techn. 1:177-197.
Rothwell, E. 1974. Manufacture and quality control of synthetic flocculants. Water Treat. Exam. 23:372-376.
McCollister, D.D., C.L. Hake, S.E. Sadek, and V.K. Rowe. 1965. Toxicological investigations of polyacrylamides. Toxicol. Appl. Pharmacol. 7:639-651.
Spray Application
Agassi, M. and M. Ben-Hur. 1992. Stabilizing steep slopes with soil conditioners and plants. Soil Technology 5:249-256.
Fox, D., and R.B. Bryan. 1992. Influence of a polyacrylamide soil conditioner on runoff generation and soil erosion: Field tests in Baringo District, Kenya. Soil Technology 5:101-119.
